What Statement Best Describes an Intensive Property
A property of a substance that does not depend on. Which statement describes an intensive property of matter.
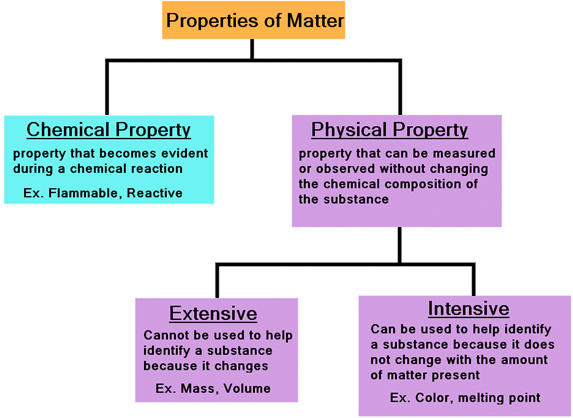
Properties Extensive And Intensive Texas Gateway
It depends on how a substance was formed c.

. A property of a substance that depends on the quantity of the substance present. Your email address will not be published. What statement best describes an intensive property.
It is the same for every sample of a single substance. It depends on the amount of substance present. It depends on the amount of substance present.
Which statement describes an intensive property of matter. It depends on how a substance was formed. It depends on how a substance was formed.
Give an example of an intensive property. These properties remain the same all the time. It depends on how a substance was formed.
Give an example of an intensive property. It depends on the amount of substance présent. It is the same for every sample of every substance.
It is the same for every sample of every substance d. It is the same for every sample of every substance. It is the same for every sample of a single substance.
It is the same for every sample of every substance D. L A Moving to another question will save this response. Intensive and Extensive Property - An intensive property is those which have the same value for any part of the system or the properties independent of the mass of the system.
Question 48 What statement best describes an intensive property. A property of a substance that depends on the mass of the substance but not the. Which of the following statements best describes the treatment of selling expenses with respect to inventories.
It is the same for every sample of every substance. Which statement describes an intensive property of matter. It depends on how a substance was formed C.
Which statement describes an intensive property of matter. A property of a. An intensive property is a property of matter that does not change as the amount of matter changes.
It depends on the amount of substance present. It depends on the amount of substance present. It is the same for every sample of every substance.
It is the same for every sample of a single substance. View Chapter_1_Practice_Test from CHEM 1102 at University of Alabama. Which statement describes an intensive property of matter 2 See answers Advertisement Advertisement arthy10122002 arthy10122002 Intensive property describes the mass and size of the property Advertisement Advertisement topanswers topanswers I hope the question is incomplete and the answer options are missing.
Scientists have developed an explanation of a phenomenon from several verified hypotheses. It is a bulk property which means it is a physical property that is not dependent on the size or mass of a sample. Which statement describes an intensive property of matter A.
Which statement describes an intensive property of matter. It depends on the amount of substance present. Leave a Reply Cancel reply.
It depends on how a substance was formed. It is the same for every sample of a single substance b. In contrast an extensive property is one that does depend on sample size.
It is the same for every sample of a single substance B. Another name for intensive properties is physical properties. An extensive property of a system depends on the system size or the amount of matter in the system.
It is the same for every sample of a single substance. An intensive property of a substance refers to that property that does not depend on the size or the quantity of that substance that is available. Examples of extensive properties include mass and volume.
A a property of a substance that does not depend on the quantity of the substance present B a property of a substance that depends on the quantity of the substance present C a property of a substance that depends on the mass of the substance but not the volume of the substance. Which statement best describes why specific heat capacity is often more useful than heat capacity for scientists when comparing two materials. Specific heat capacity is an intensive property and does not depend on sample size.
The explanation has been confirmed through numerous experimental testswhich option best describes this explanation. Chapter 1 Practice Test What statement best describes an intensive property.

Density An Intensive Property Of Matter Carolina Com

Examples Of Intensive Extensive Properties Of Matter Video Lesson Transcript Study Com
What Are Some Examples Of Extensive And Intensive Properties Quora
Comments
Post a Comment